Pharmaceutical & Medical CMS
For Medical and Pharmaceutical firms seeking innovative ways to lower the cost of governance, compliance, and documentation.
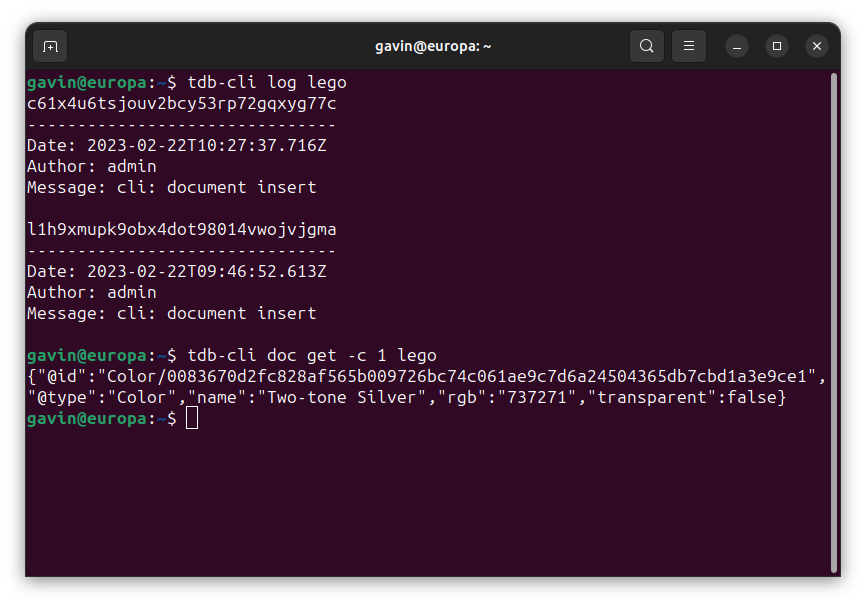
TerminusCMS at a Glance
An open-source solution for the challenges of medical and pharmaceutical firms. Here is a quick look at feature highlights, more details about the challenges, solutions, and benefits of TerminusCMS are listed below.
GraphQL API
With a single GraphQL endpoint, use an API query language you know to create frontends for internal and external users and customers.
Change Request Workflows
Collaboration, QA, and approval workflows to fit the methodologies of your teams
Revision Control
Data and content are immutable giving you the ability to branch, merge, diff, rollback, and time-travel
Analytics Engine
AI/ML semantic CMS capabilities when you couple GraphQL with Datalog in our analytics engine
Content & Data Collaboration & Federation
Integrate data, compare versions of content, automate approval processes, and work collaboratively
NOW & FOREVER
Challenges
Pharmaceutical and medical companies are complex operations. From discovery to launch, there are vast amounts of data, documentation, and content required for researchers, medical professionals, patients, and legislative bodies. TerminusCMS is designed for complex content infrastructures, helping with challenges such as –
Data Sharing and Collaboration
Cross-departmental data and content sharing due to disparate applications and methodologies
Surfacing Clinical Trial Data
Clinical trial results often get published in medical papers and journals making it difficult for medical professionals to access and absorb
External Research Discovery
Access to research and external data is a slow process impacting researchers and the discovery team’s productivity
Federating Content for All Users
Content, data, and assets are required for patients, medical professionals, and internal teams, this process is disjointed making it time consuming and costly
Lower the Cost of Compliance
Pharma & Medical companies are content and data-rich with important information required for governance and regulatory compliance. Without semantic connections discovering and using this information is a costly and time consuming challenge.
Solutions
TerminusCMS semantically connects and federates content in complex environments. Deliver user-relevant information to an array of frontends with GraphQL. The analytics engine makes data, content, and assets discoverable and reusable. Here are some of the solutions to the pharmaceutical and medical content challenges.
Change Request Workflows
Change request workflows and approval processes for to ensure accuracy of inputted data and documentation.
Use Your History
TerminusCMS is immutable and all changes are saved as deltas. See how development evolves. Its native revision control lets you roll back, time-travel, and compare any data or content throughout its lifecycle
Clinical Trial Results as Content
Researchers can use TerminusCMS to collate trial results and publish them for others to query and use as content
Bring External Data In
Ingest external data and papers and create semantic connections to help researchers find the information to aid and support their work
Content Federation
The revision-control features of TerminusCMS include push, pull, and clone, time travel, rollback, and the ability to compare two documents from any point of its lifecycle
EMA & FDA Applications
Support EMA and FDA applications by modeling application requirements to automate and speed up the documentation process
Analytics Engine
AI/ML semantic CMS capabilities when you couple GraphQL with Datalog in our analytics engine to make data and content queryable
Benefits
TerminusCMS helps you connect teams, data, and content to improve your productivity, stakeholder comms, and lower the cost of managing and publishing content
Faster Discovery and Development
Make external data and papers quicker to discover and use. Capture data about shortlisted compound tests such as dosage, side effects, and usage to reuse as content. Turn test data into information for the preclinical research team to hit the ground running
Better Patient Care
Enable medical professionals to query clinical trial results to determine the best fit for their patient demographics
Efficient EMA/FDA Application and Review
Create content models to automatically generate the backbone of your applications using the data and content generated from the discovery, development, and trial phases
Lower Content Production Costs
TerminusCMS reduces duplication of work by connecting teams, federating content, and making it reusable. It is open-source and available to extend without limits saving considerable software licensing fees
Reduce Technical Debt & Complexity
Managing the data and content requirements across teams, applications, and ELT pipelines is costly and time-consuming. Reduce your technical debt with TerminusCMS


Start Free
Get started in minutes and for free with our TerminusCMS Community Package. Clone an example from the dashboard to experiment and play today.